
The Sarah Cannon Research Institute, based in Nashville, received nearly $8 million in payments from drug companies on behalf of its president for clinical operations, Dr. Howard Burris, largely for research work. Dozens of his articles published in prestigious medical journals did not include the required disclosures of those payments and relationships. – William DeShazer for The New York Times
“Calls for transparency stem from concerns that researchers’ ties to the health and drug industries increase the odds they will, consciously or not, skew results to favor the companies with whom they do business.”
At this point in the medical news stream, this is a big Duh, to anyone with eyes open, who has read about this subject in the last oh, I dunno, twenty years, It is why the onus of responsibility, and making an intelligently calculated risk decision of whether to take (especially newly marketed) medication, or agree to any procedure, or have surgery, is on us, the patient.
Trust your doctor, if you can, but, if you don’t know the bigger reality in the business of healthcare, you could suffer needlessly.
By Charles Ornstein and Katie Thomas, Via NYTimes
Dec. 8, 2018
This article was reported and written in collaboration with ProPublica, the nonprofit journalism organization.
One is dean of Yale’s medical school. Another is the director of a cancer center in Texas. A third is the next president of the most prominent society of cancer doctors.
These leading medical figures are among dozens of doctors who have failed in recent years to report their financial relationships with pharmaceutical and health care companies when their studies are published in medical journals, according to a review by The New York Times and ProPublica and data from other recent research.
Dr. Howard A. “Skip” Burris III, the president-elect of the American Society of Clinical Oncology, for instance, declared that he had no conflicts of interest in more than 50 journal articles in recent years, including in the prestigious New England Journal of Medicine.
However, drug companies have paid his employer nearly $114,000 for consulting and speaking, and nearly $8 million for his research during the period for which disclosure was required. His omissions extended to the Journal of Clinical Oncology, which is published by the group he will lead.
In addition to the widespread lapses by doctors, the review by The Times and ProPublica found that journals themselves often gave confusing advice and did not routinely vet disclosures by researchers, although many relationships could have been easily detected on a federal database.
Medical journals, which are the main conduit for communicating the latest scientific discoveries to the public, often have an interdependent relationship with the researchers who publish in their pages. Reporting a study in a leading journal can heighten their profile — not to mention that of the drug or other product being tested. And journals enhance their cachet by publishing exclusive, breakthrough studies by acclaimed researchers.
In all, the reporting system still appears to have many of the same flaws that the Institute of Medicine identified nearly a decade ago when it recommended fundamental changes in how conflicts of interest are reported. Those have yet to happen.
“The system is broken,” said Dr. Mehraneh Dorna Jafari, an assistant professor of surgery at the University of California, Irvine, School of Medicine. She and her colleagues published a study in August that found that, of the 100 doctors who received the most compensation from device makers in 2015, conflicts were disclosed in only 37 percent of the articles published in the next year. “The journals aren’t checking and the rules are different for every single thing.”
Calls for transparency stem from concerns that researchers’ ties to the health and drug industries increase the odds they will, consciously or not, skew results to favor the companies with whom they do business. Studies have found that industry-sponsored research tends to be more positive than research financed by other sources. And that in turn can sway which treatments become available to patients. There is no indication that the research done by Dr. Burris and the other doctors with incomplete disclosures was manipulated or falsified.
Journal editors say they are introducing changes that will better standardize disclosures and reduce errors. But some have also argued that since most researchers follow the rules, stringent new requirements would be costly and unnecessary.
The issue has gained traction since September, when Dr. José Baselga, who was the chief medical officer of Memorial Sloan Kettering Cancer Center in New York, resigned after The Times and ProPublica reported that he had not revealed his industry ties in dozens of journal articles.
[Read more about doctors at Memorial Sloan Kettering and their financial relationships with companies.]
Dr. Burris, president of clinical operations and chief medical officer at the Sarah Cannon Research Institute in Nashville, referred questions about the payments to his employer. It defended him, saying the payments were made to the institution, although The New England Journal of Medicine requires disclosure of all such payments.
Other prominent researchers who have submitted erroneous disclosures include Dr. Robert J. Alpern, the dean of the Yale School of Medicine, who failed to disclose in a 2017 journal article about an experimental treatment developed by Tricida that he served on that company’s board of directors and owned its stock. Tricida, which is developing therapies for chronic kidney disease, had financed the clinical trial that was the subject of the article.
Dr. Alpern said in an email that he initially believed that his disclosure — that he had been a consultant for Tricida — was adequate. However, “because of concerns recently raised about disclosures,” he said he notified the publication, The Clinical Journal of the American Society of Nephrology, in October that he also served on Tricida’s board and had stock holdings in the company.
The journal initially told Dr. Alpern that his disclosure was sufficient. But after The Times and ProPublica contacted the publication in November, it said it would correct the article.
“The failure to disclose this information at the time of peer review is a violation of our policy,” Dr. Rajnish Mehrotra, the journal’s editor in chief, said in an email.
He later said that an additional inquiry had revealed that all 12 of the article’s authors had submitted incomplete disclosures, and that the journal planned to refer the matter to the ethics committee of the American Society of Nephrology. Dr. Mehrotra also said that the journal had decided to conduct an audit of some recent articles to evaluate the broader issue.
Dr. Carlos L. Arteaga, the director of the Harold C. Simmons Comprehensive Cancer Center in Dallas, said he had “nothing to disclose” as an author of a 2016 study published in The New England Journal of Medicine of the breast cancer drug Kisqali, made by Novartis. But Dr. Arteaga had received more than $50,000 from drug companies in the three-year disclosure period, including more than $14,000 from Novartis.
In an email, Dr. Arteaga described the omission as an “inexcusable oversight and error on my part,” and subsequently submitted a correction.
Dr. Jeffrey R. Botkin, an associate vice president for research at the University of Utah, recently argued in JAMA, a leading medical journal, that researchers should face misconduct charges when they do not disclose their relationships with interested companies. “They really are falsifying the information that others rely on to assess that research,” he said. “Money is a very powerful influencer, and people’s opinions become subtly biased by that financial relationship.”
But Dr. Howard C. Bauchner, the editor in chief of JAMA, said that verifying each author’s disclosures would not be worth the time or effort. “The vast majority of authors are honest and do want to fulfill their obligations to tell readers and editors what their conflicts of interest could be,” he said in an interview.
As the debate continues, an influential group, the International Committee of Medical Journal Editors, is considering a policy that would refer researchers who commit major disclosure errors to their institutions for possible charges of research misconduct.
Concerns about the influence of drug companies on medical research have persisted for decades. Senator Estes Kefauver held hearings on the issue in 1959, and there was another surge of concern in the 2000s after a series of scandals in which prominent doctors failed to reveal their industry relationships.
Medical journals and professional societies strengthened their requirements. The drug industry restricted how it compensates doctors, prohibiting gifts like tickets to sporting events or luxury trips — although evidence of kickbacks and corruption continues to surface in criminal prosecutions. And a 2010 federal law required pharmaceutical and device makers to publicly report their payments to physicians.

Questioned about omissions on his disclosure forms accompanying research articles, Dr. Burris submitted new disclosures to the New England Journal of Medicine showing his ties to many drug and health care companies.
Despite these changes, the system for disclosing conflicts remains fragmented and weakly enforced. Medical journals and professional societies have a variety of guidelines about what types of relationships must be reported, often leaving it up to the researcher to decide what is relevant. There are few repercussions — beyond a correction — for those who fail to follow the rules.
For example, the American Association for Cancer Research has warned authors that they face a three-year ban if they are found to have omitted a potential conflict. But the group’s policy on conflicts of interest contains no mention of such a penalty, and it said no author had ever been barred. Dr. Baselga’s failure to disclose his industry relationships extended to the association’s journal, Cancer Discovery, for which he serves as one of two editors in chief. The association said it is investigating Dr. Baselga’s actions.
Most authors do seem to disclose their ties to corporate interests. About two-thirds of the authors on the Kisqali study, for example, reported relationships with companies, including Novartis. But the researchers who did not included Dr. Arteaga, Dr. Burris and Dr. Denise A. Yardley, a senior investigator who works with Dr. Burris at Sarah Cannon.
The Tennessee-based research center received more than $105,000 in fees for consulting, speaking and other services on Dr. Yardley’s behalf in the three-year period in which she declared no conflicts.
The Sarah Cannon institute said it switched over a year ago to a “universal disclosure” practice promoted by ASCO, the cancer group that Dr. Burris will lead. That requires doctors to disclose all payments, including those made to their institutions.
“We believe we adhere to the highest ethical standards in the industry by not allowing personal compensation to be paid to our leadership physicians,” the center said.
ASCO said it would post corrections to Dr. Burris’s disclosures in The Journal of Clinical Oncology for the past four years. The group said that in the fall of 2017 — as Dr. Burris was seeking a leadership role in the organization — it began working with him to disclose all his company relationships, including indirect payments. Dr. Burris will become president in June 2019.
“Disclosure systems and processes in medicine are not perfect yet, and neither are ASCO’s,” the group said in an email.
Dr. Burris, Dr. Yardley and Dr. Arteaga submitted updated disclosures to The New England Journal of Medicine, which posted them on Thursday.
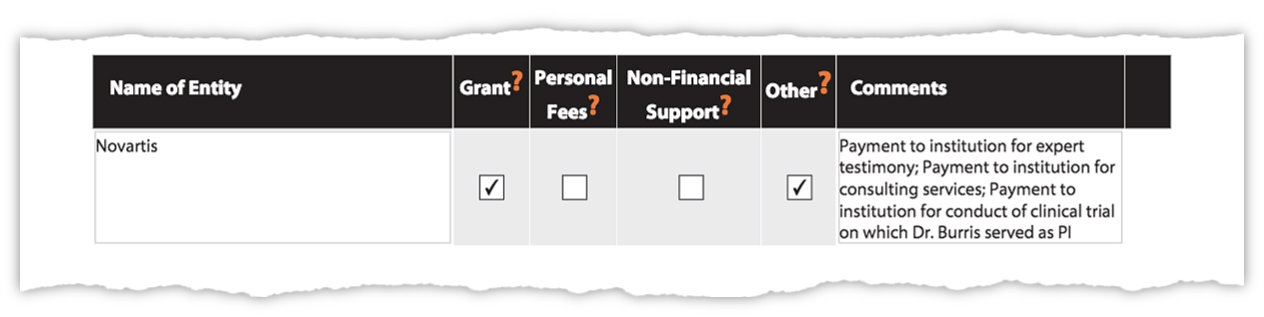
Dr. Burris’s new corrections to his disclosure forms that were posted by the New England Journal of Medicine show payments to his employer from Novartis, among other companies, for his work.
Dr. Burris’s updated disclosure listed relationships with 30 companies, including that he provided expert testimony for Novartis.
Other studies recently published by the New England Journal of Medicine also omitted disclosures, including one on a 2018 study on a treatment for sickle cell disease and another on the recently approved cancer drug Vitravki, to be sold by Bayer and Loxo Oncology.
Jennifer Zeis, a spokeswoman for the journal, said that it was contacting those studies’ authors, and that it now asked researchers to certify that they had checked their disclosures against the federal database.
Some institutions have pushed back, arguing that the journals’ inconsistent rules make it difficult for even well-meaning researchers to do the right thing.
In a letter last month To the New England Journal of Medicine, Memorial Sloan Kettering objected to the treatment of one of its top researchers, Dr. Jedd Wolchok. When he tried to correct his disclosures, the journal shifted its position, from saying its editors were satisfied with his disclosures to saying he had failed to comply with the rules, the center said in citing communications with the journal.
Dr. Wolchok, a pioneer in cancer immunotherapy, ultimately corrected 13 articles and letters to the editor.
To clarify reporting requirements, several publications are attempting only now to do what the Institute of Medicine recommended in 2009. The New England Journal is testing a new system in partnership with the Association of American Medical Colleges that would act as a central repository for reporting financial relationships.
This year, JAMA began requiring authors to confirm multiple times that they had nothing to disclose. ASCO has a centralized system for reporting conflicts to all of its journals and speaker presentations.
Dr. Bernard Lo, the chairman of the 2009 Institute of Medicine panel, said journals have only begun to confront some of the systemic flaws. “They’re certainly not out in front trying to be trailblazers, let me just say it that way,” he said. “The fact that it hasn’t been done means that nobody has it on their priority list.”
Charles Ornstein is a senior editor at ProPublica.

